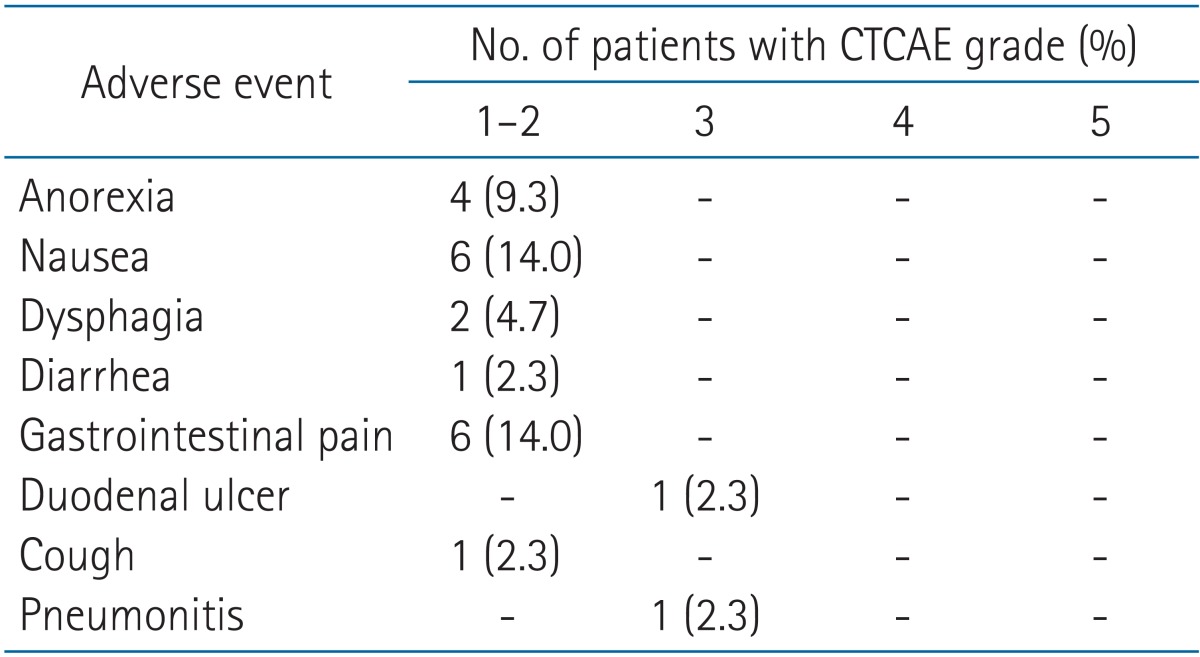
A review of criteria strictness in “Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials” - ScienceDirect

Composite grading algorithm for the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) - Ethan Basch, Claus Becker, Lauren J Rogak, Deborah Schrag, Bryce B
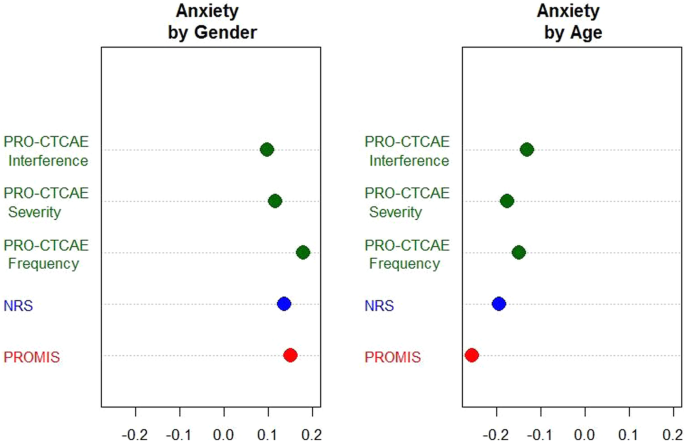
Establishing a common metric for patient-reported outcomes in cancer patients: linking patient reported outcomes measurement information system (PROMIS), numerical rating scale, and patient-reported outcomes version of the common terminology criteria ...

Table 4 from The use of and adherence to CTCAE v3.0 in cancer clinical trial publications | Semantic Scholar
Using the Skindex-16 and Common Terminology Criteria for Adverse Events to assess rash symptoms: results of a pooled-analysis (N0993) - Document - Gale Academic OneFile
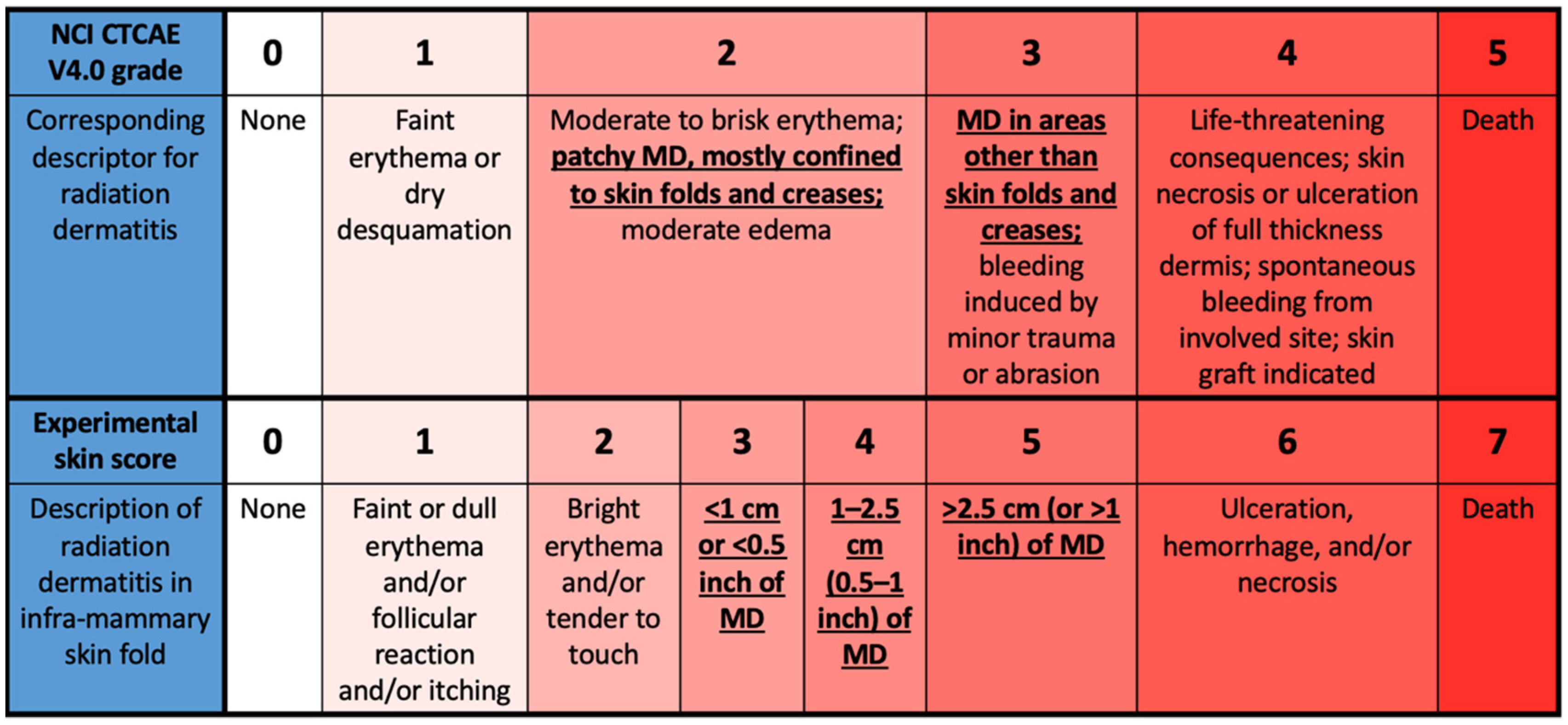
Current Oncology | Free Full-Text | Validation of a Patient-Reported Outcome Measure for Moist Desquamation among Breast Radiotherapy Patients

Evaluation of the patient experience of symptomatic adverse events on Phase I clinical trials using PRO-CTCAE | British Journal of Cancer
![PDF] Validation of the German patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE™). | Semantic Scholar PDF] Validation of the German patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE™). | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e89232b4d6b07e0d806120c23e8df3645e4acf70/3-Table1-1.png)



![Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF](https://image.slidesharecdn.com/ctcae4-022009-09-15quickreference8-5x111-120711021618-phpapp01/85/ctcae-402-20090915quickreference85x111-2-320.jpg?cb=1668437655)






![Grading scales of the CRS Penn Grading Scale [20] CTCAE v5.0 [80]... | Download Scientific Diagram Grading scales of the CRS Penn Grading Scale [20] CTCAE v5.0 [80]... | Download Scientific Diagram](https://www.researchgate.net/publication/334158848/figure/tbl2/AS:852086526844929@1580164667382/Grading-scales-of-the-CRS-Penn-Grading-Scale-20-CTCAE-v50-80-Lee-MDACC-16.png)