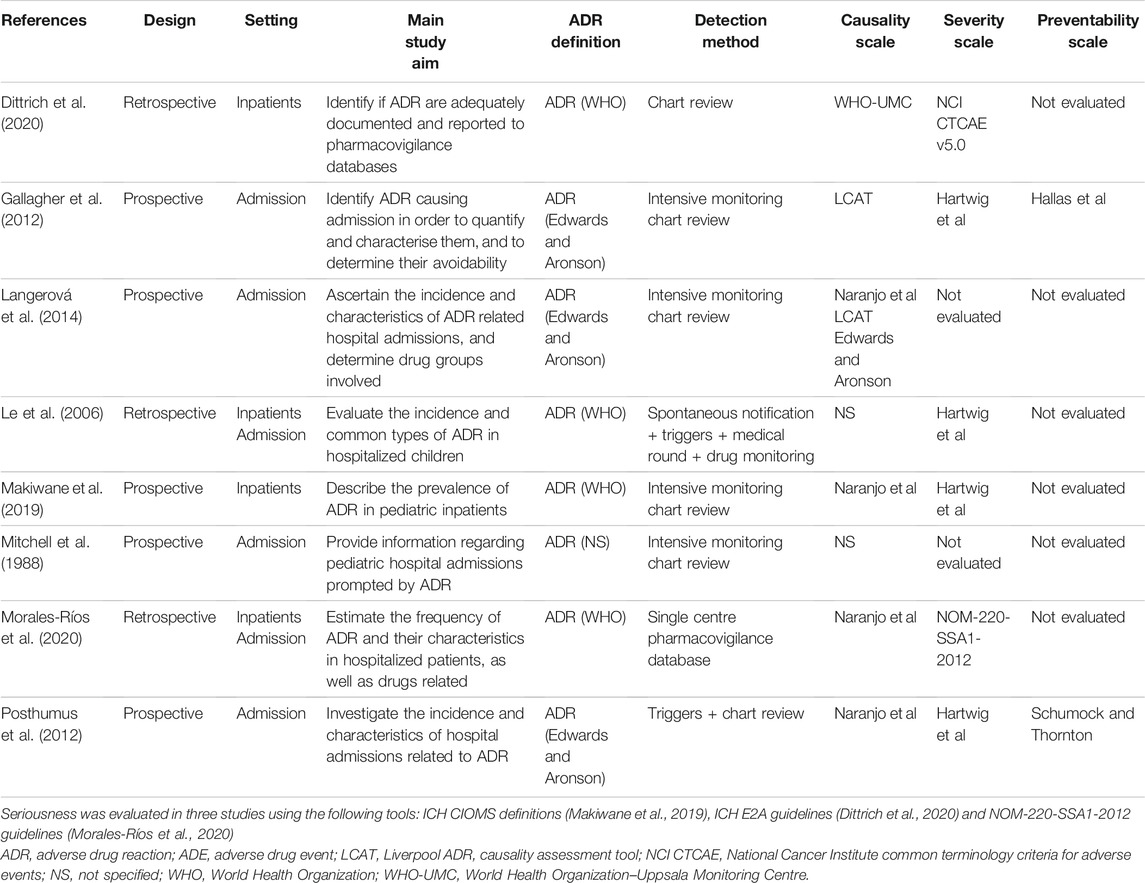
Table 1 from SMARTS (Systematic Monitoring of Adverse events Related to TreatmentS): The development of a pragmatic patient-completed checklist to assess antipsychotic drug side effects | Semantic Scholar
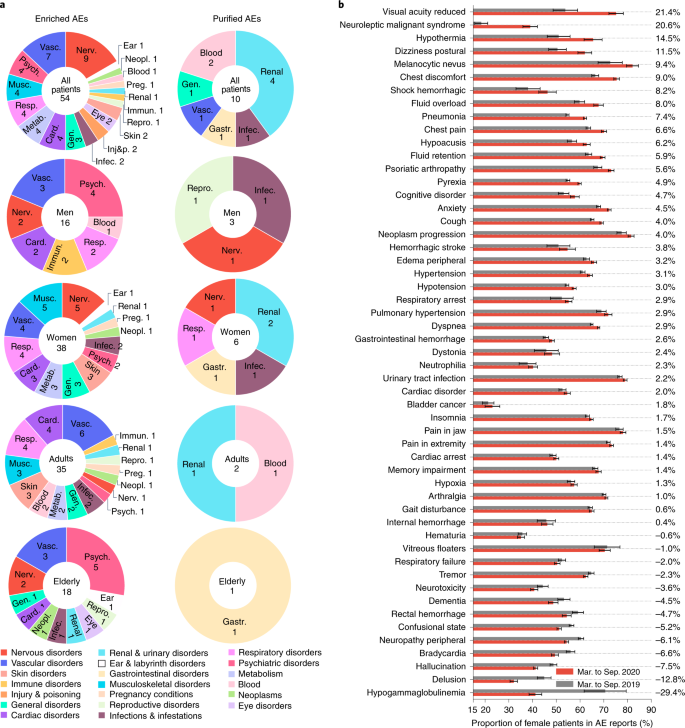
Population-scale identification of differential adverse events before and during a pandemic | Nature Computational Science
Adverse events from systemic treatment of cancer and patient-reported quality of life | MDedge Hematology and Oncology
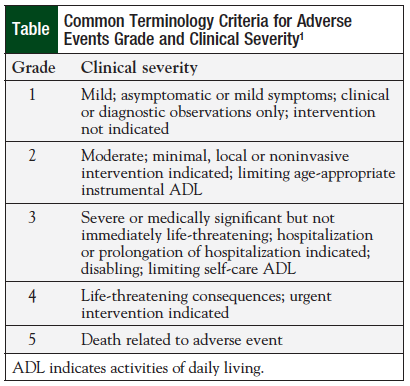
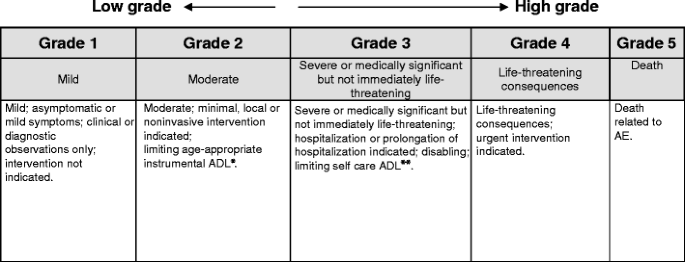
Adverse-event severity definitions and how they were measured with the... | Download Scientific Diagram

From implementation to sustainment: A large-scale adverse event disclosure support program generated through embedded research in the Veterans Health Administration
Reporting of Clinical Adverse Events Scale: a measure of doctor and nurse attitudes to adverse event reporting. - Leeds Beckett Repository
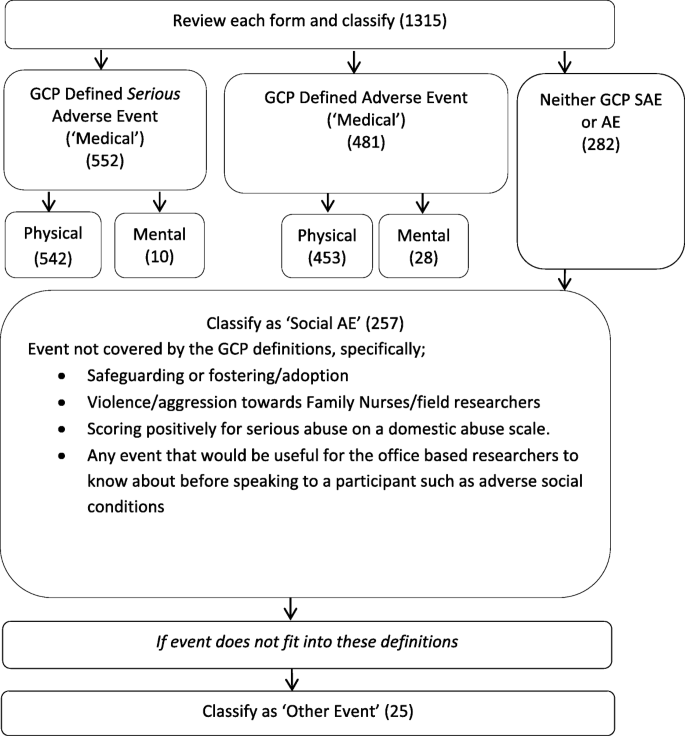
Monitoring adverse social and medical events in public health trials: assessing predictors and interpretation against a proposed model of adverse event reporting | Trials | Full Text

Population-scale identification of differential adverse events before and during a pandemic | Nature Computational Science

Inter-rater reliability of the neonatal adverse event severity scale using real-world Neonatal clinical trial data | Journal of Perinatology
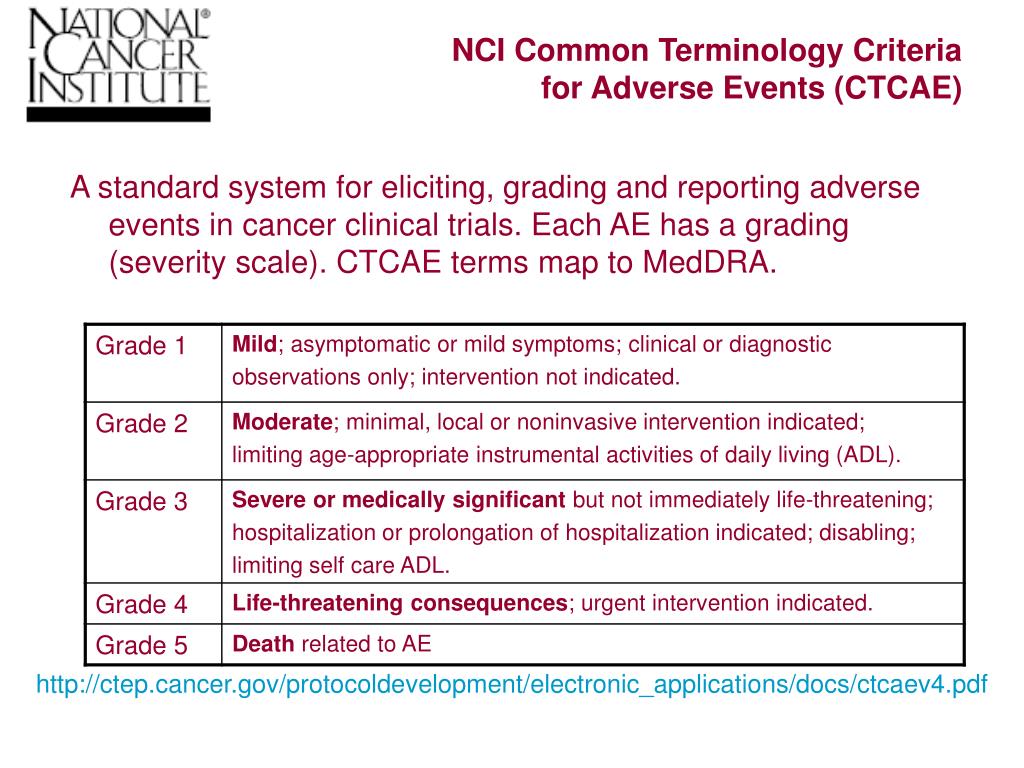
A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group | Supportive Care in Cancer

Composite grading algorithm for the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) - Ethan Basch, Claus Becker, Lauren J Rogak, Deborah Schrag, Bryce B